Abstract:
Nanotechnology is having a big impact on pharmaceutical sciences, and drug delivery systems are one area where this is most evident. Compared to conventional medication delivery methods, nanoparticles provide a number of benefits, including increased effectiveness and fewer adverse drug reactions. This article provides a concise overview of advances in nanotechnology & highlights their transformative potential in modern medicine.
Introduction
Drug delivery is now entering a new era with nanotechnology, which enables the design of nanoscale structures with sizes between 0.1 and 100 nm for precise and targeted therapy. These structures can then be further developed into complex devices with particular characteristics [1]. Systems that offer special benefits in drug encapsulation and delivery include micelles, liposomes, dendrimers, carbon nanotubes, metallic nanoparticles, and quantum dots. With the goal of killing specified cells and minimizing side effects from drug distribution in non-target locations, NP-based drug delivery systems are made to deliver medication directly to a particular area of the body.
Advancements in Pharmaceutical Nanotechnology
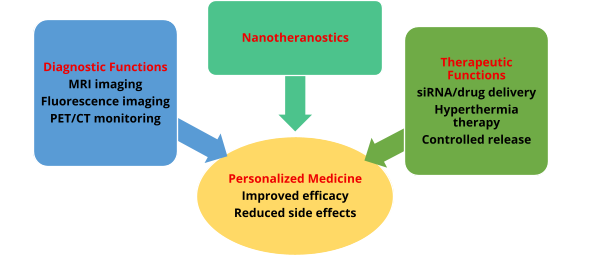
1 Nanotheranostics
"Theranostics" refers to the simultaneous integration of diagnosis and treatment [2]. The aim is to create tailored therapeutic approaches for personalized medicine, considering that high efficacy from specific treatments can only be achieved for a small number of patients.
Research-backed examples
To deliver small interfering RNA (siRNA) to tumor cells with a concurrent hyperthermia-based therapy, Park et al. [3] reported a unique synthesis, a comprehensive in vitro evaluation, and the use of magnetic iron cobalt nanoparticles (as a core) and graphitic carbon (as a shell). Together, these tactics stopped tumor cells from proliferating and caused them to undergo apoptosis.
Elsherbini et al. [4] reported a similar application of hyperthermia in which Fe₃O₄ NPs were used to simultaneously raise the temperature to 47°C under radiofrequency exposures at 25 kW; at this point, the apoptotic cells' monitoring showed dark signal intensity in the longitudinal relaxation time (T1)-weighted images, as examined in Ehrlich tumours.
Furthermore, the production of sugar-coated iron oxide nanoparticles specifically intended as negative contrast agents for magnetic resonance imaging (MRI) and heat mediators for magnetic fluid hyperthermia has been reported [5].
PEG can increase the solubility of nanocarriers due to its hydrophilicity, decrease kidney clearance due to an increased hydrodynamic size of the PEG-carrier conjugate, and protect the core of nanocarriers from degradation by steric hindrance.
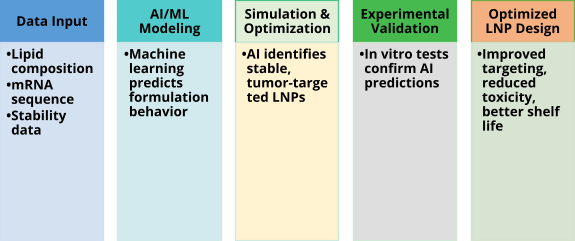
2 AI in Nanotechnology
The development of cancer vaccines has been revolutionized by the convergence of nanomedicine and artificial intelligence (AI), especially in the optimization of RNA-loaded lipid nanoparticles (LNPs). By making predictive modeling of multi-parametric interactions possible, AI changed conventional nanomedicine operations [6].
This is crucial for solving two major challenges:
Ⅰ. Stability: Approaches driven by AI can be used to forecast lipid packing density and adjust cryoprotectant ratios, which could decrease cold chain dependencies while increasing shelf life.
Ⅱ. Targeting: The payoff is exceptional precision. AI-optimized LNPs have demonstrated a dramatic ability to accumulate in tumors (up to 89% improvement in models) while minimizing off-target delivery to the liver to less than 5% [7]. More medication then reaches the target area, resulting in fewer adverse reactions. Researchers can now create more intelligent and efficient nanocarriers significantly faster using AI technology.
3 Stimuli-Responsive Nanosystems
The nanocarriers are primarily designed to deliver, release, and activate cargos in specific locations, including tumor microenvironments or the intracellular spaces within cancer cells. They respond to internal or external stimuli, such as pH, enzymes, redox potential, temperature, and magnetic fields [8, 9].
In addition, external stimuli can also trigger biological responses in nanocarriers, such as an external magnetic field, potentially increasing the accumulation of magnetic nanocarriers in tumors. The stimuli could also be used to trigger biological responses in prodrug-formulated nanocarriers within diseased areas/cells, enabling precision treatment. The stimuli-responsive nanocarriers were found to be effective in overcoming multidrug resistance in cancer treatment [10].
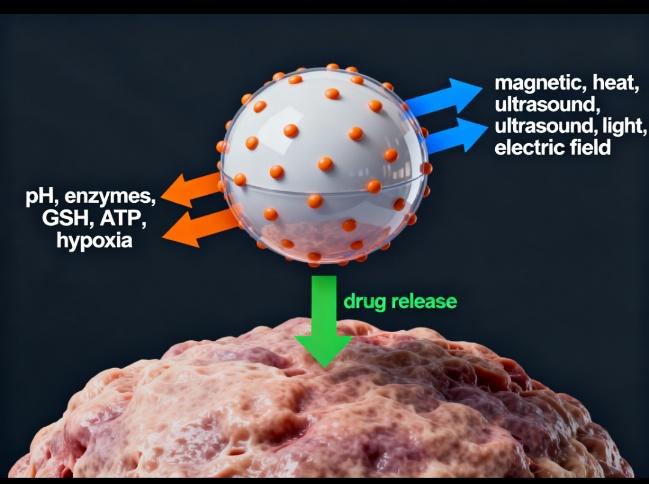
Table 1: Key Internal and External Stimuli for Stimuli-Responsive Nanocarriers
Stimuli Type | Trigger | Mechanism | Examples |
Internal Stimuli | pH | Acidic tumor microenvironment triggers drug release | Doxorubicin-loaded pH-sensitive liposomes |
Enzyme | Enzyme cleavage of polymer linkers releases drug | Matrix metalloproteinase (MMP)-responsive polymeric micelles | |
Redox | High intracellular glutathione reduces disulfide bonds | Redox-sensitive paclitaxel micelles | |
Hypoxia | Hypoxia-activated prodrugs release under low O₂ | Tirapazamine-conjugated nanoparticles | |
External Stimuli | Magnetic Field | Magnetic guidance enhances tumor accumulation | Magnetite (Fe₃O₄) nanocarriers for targeted delivery |
Temperature | Heat increases membrane permeability and drug diffusion | Thermosensitive liposomes with doxorubicin |
Challenges and Future Perspectives
Things have improved, but problems still exist. Some materials may not be safe enough, and the gap between laboratory results and actual biological tests remains a significant concern. Data quality and the "black box" nature of complicated models, which lack transparency, are major challenges in AI-driven design. AI still faces difficulties with model transparency and data quality. To advance in the future, interdisciplinary collaboration is necessary to utilise AI in reducing unintended side effects and predicting long-term safety risks, thereby enabling the development of clinically viable nanomedicines [11].
Conclusion
Nanotechnology represents a highly promising science offering a wide range of benefits and applications within the medical sector. Innovations and AI are pretty much going to drive the next big changes in personalized medicine. As technology and AI improve, they’ll help steer personalized medicine into its next stage.


