Why Lithium-Metal Batteries Matter
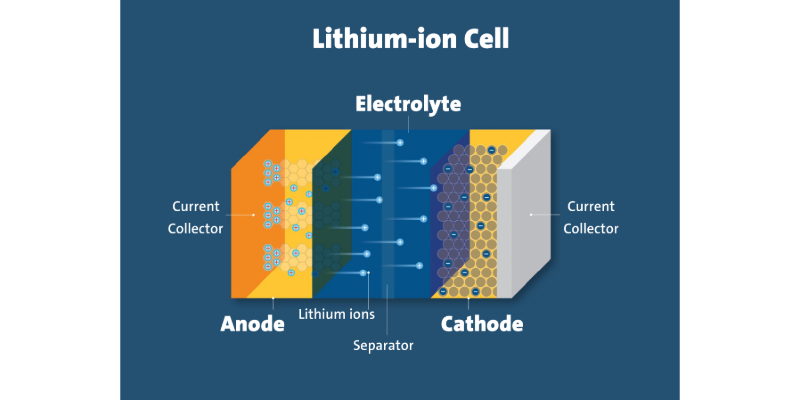
Lithium-metal batteries, equipped with lithium metal anodes, have the remarkable ability to store over twice the energy of traditional lithium-ion batteries with graphite anodes. Despite this potential, most battery-powered devices continue to rely on less advanced lithium-ion technology. Recently, chemists at Brookhaven made groundbreaking strides in enhancing lithium-metal batteries for the Department of Energy (DOE) by incorporating a compound called cesium nitrate into the electrolyte that separates the battery's anode and cathode. This strategic addition effectively targeted the interphase, a vital protective layer on the battery's electrodes that significantly influences the number of charge and discharge cycles a battery can endure. With the cesium nitrate additive, these advanced batteries can recharge more rapidly while preserving their cycle life.
However, detailed analysis conducted with cutting-edge tools at the National Synchrotron Light Source II and the Center for Functional Nanomaterials unveiled two unexpected outcomes: a previously unknown component in the interphase and the absence of a factor once thought essential for optimal battery performance. These insights not only challenge long-standing assumptions about battery technology but also pave the way for exciting new opportunities in battery engineering.